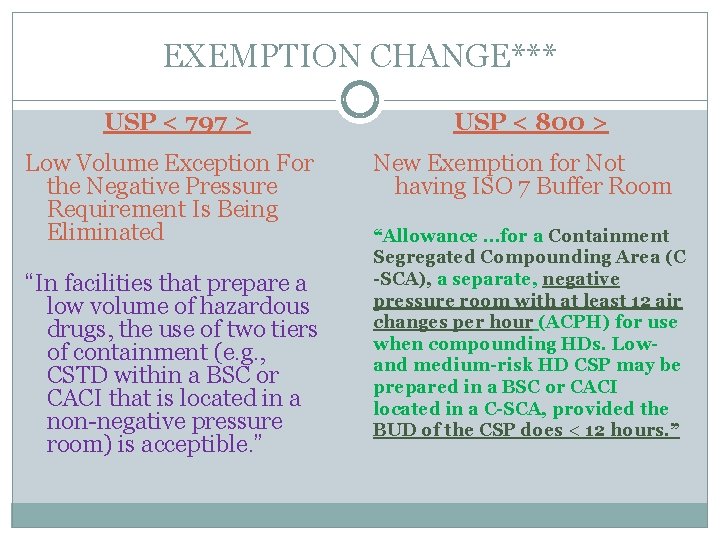
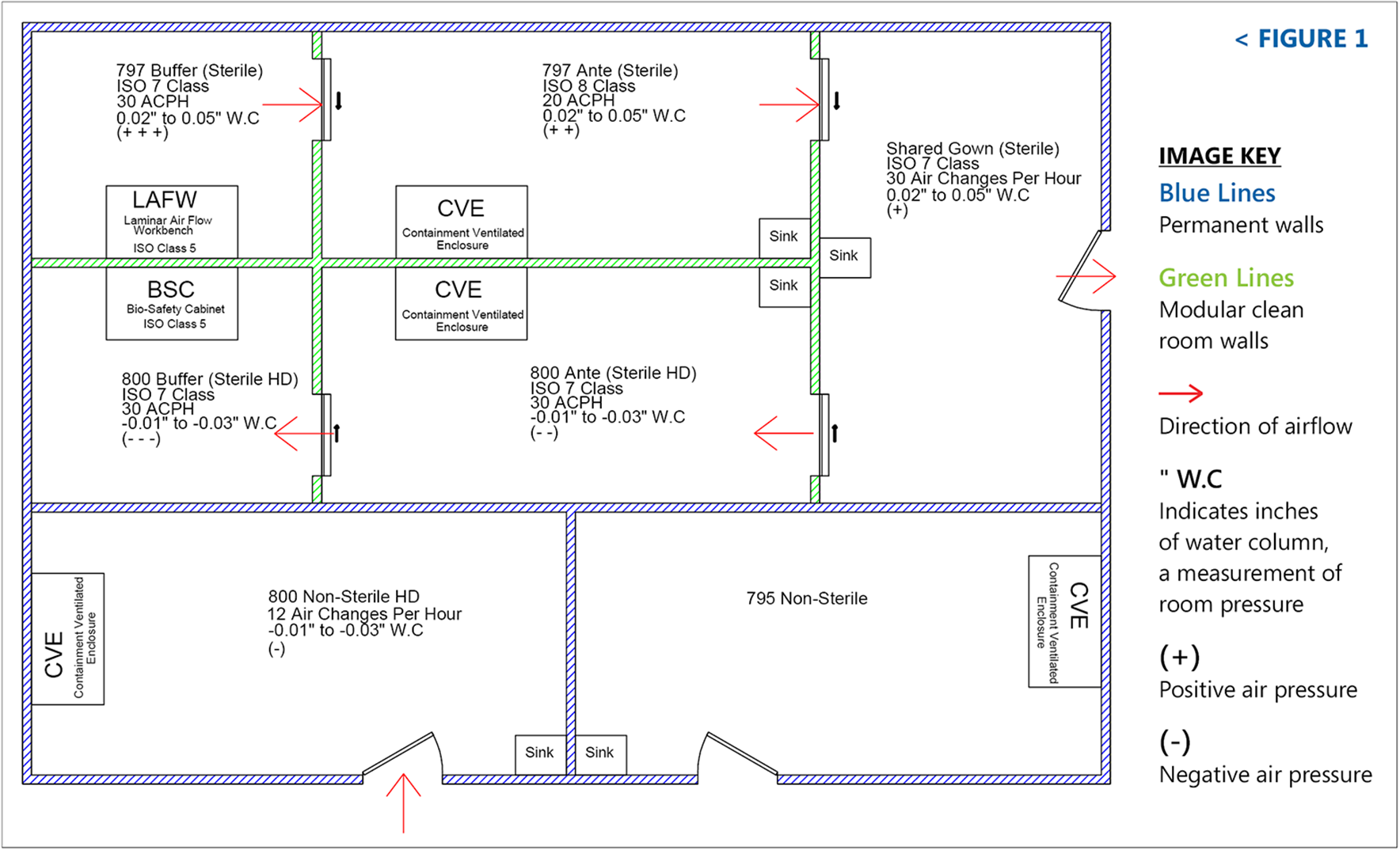
usp 797 cleaning guidelines sorted by
relevance
-
Related searches:
- bilder von mädels
- hautausschlag bauch und rücken
- dolly buster porn pics
- plavi oglasnik zagreb osobni kontakti
- dhating naach mp3 songs pk download
- liebt er eine andere tarot
- Yulia Peresild nackt
- Jasmine Lia nackt
- liebessprüche guten morgen
- suche geile hausfrau
- smiley rote bäckchen
- alt jung ficken
- who is heather locklear
- hentai pov gif
- nackte frauen ärsche

Admin09.08.2021
4403

Admin04.08.2021
7508